1. Understanding How Detergent Works
To know why cleaning efficiency falls off, it’s important to understand how cleaning works. Industrial parts washing relies heavily on the chemical action of soaps and detergents to remove oils, greases, and particulate soils from metal components. To understand the need for filtration, it’s essential to first understand how detergent does its job.
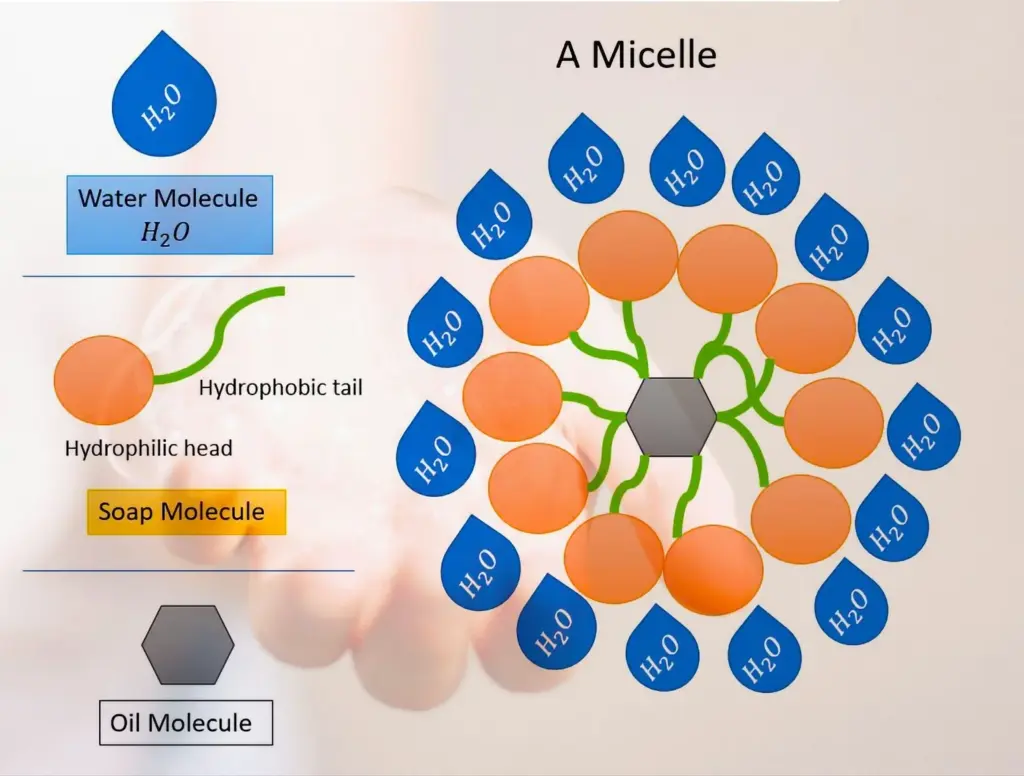
Detergent Molecule Structure
Detergent is a type of surfactant, meaning it reduces the surface tension between water and other substances like oils or solids. Each detergent molecule has two ends:
- A hydrophilic “head” that is water-attracting.
- A hydrophobic “tail” that is oil-attracting and repels water.
This dual nature allows detergent to act as a bridge between water and oil, which would otherwise not mix.
The Cleaning Process
The cleaning action of detergent involves several key steps:
- Lowering Surface Tension: Detergent reduces water’s surface tension, allowing it to spread and penetrate more effectively.
- Micelle Formation: The hydrophobic tails of detergent molecules bind to oils and greases, while the hydrophilic heads remain in the water. This forms micelles—tiny spherical structures that encapsulate and trap the contaminants.
- Suspension and Removal: The micelles keep the contaminants suspended in solution so they can be rinsed away, completing the cleaning process.
In an industrial washer, this mechanism—amplified by heat and mechanical agitation—is what allows for efficient cleaning of complex parts and components.
2. When Cleaning Baths Fail
Industrial cleaning systems are only as effective as the condition of the wash bath. Over time, cleaning performance deteriorates. This happens for two main reasons:
(a) Depletion of Active Detergent
As parts are cleaned, the detergent molecules form micelles around the grease, oil, and other contaminants. Eventually, all available detergent is used up—bound to grime—and no longer available to form new micelles. As this happens, the bath becomes chemically saturated and loses cleaning power.
(b) Contamination with Demulsified or Unemulsified Oils
Not all oils & greases are bound up in micelles. Some float to the surface. Some chemistries, either by design or not, release oil from micelles over time, allowing it to float free. If not skimmed, collected, or otherwise controlled (e.g., with a weir and sparger), these oils can redeposit on the parts as they are raised through this layer.
(c) Contamination with Insolubles
Industrial environments generate large amounts of solid debris, such as:

- Metal shavings or dust
- Pigments
- Rust particles
- Carbonized deposits
- Hardened residues, such as glues or dried ink
Much like washing mud off your hands into a sink, these insolubles accumulate in the bath and reduce the efficiency of the cleaning system in several ways:
- They physically block or clog spray nozzles and pumps.
- They coat parts and reduce contact between detergent and surface.
- They re-deposit on parts, creating a dirty surface
- They increase wear on system components such as seals, reducing uptime and service life.
3. The Role of Filtration in Maintaining Cleaning Performance
Filtration is the frontline defense against bath degradation. It extends the life of the cleaning solution and maintains high washing performance by addressing both causes of bath failure.
Removing Insolubles
A well-designed filtration system—often incorporating bag filters, magnetic traps, or oil skimmers—removes suspended solids continuously. This keeps the solution cleaner, prevents redeposition on parts, and protects mechanical components of the washer.
Best practice is to run fluid through a filter continuously during operation, usually using a bypass on the agitation pump. Oil skimmers work best in calm fluid, so running them overnight while the washer is not in use is preferable.
Supporting Detergent Efficiency
By keeping the bath clear of interfering debris, filtration ensures that the available detergent molecules are used where they are needed: bonding with oils and contaminants on parts. This maintains high micelle-forming efficiency for longer durations.
The Outcome – Reducing Downtime and Chemical Costs
So what does this mean for your operations? Regular filtration results in:
- Less frequent bath changeover with associated treatment/hauling costs
- Reduced detergent and water consumption
- Improved system uptime and throughput – changing a large bath (cooldown, change, refill, reheat) can take an entire day
4. Conclusion
The science of detergent and the practice of filtration go hand-in-hand in industrial parts washing. Understanding how surfactants form micelles to trap grease highlights the critical role of bath maintenance. When soap is depleted or contaminants overwhelm the system, cleaning fails, and downstream processes or customers suffer.
Filtration is not just a maintenance feature—it’s a core component of process efficiency and quality control. By incorporating high-performance filtration strategies, facilities can extend bath life, reduce environmental footprints, cut costs, and ensure consistent cleaning performance that meets modern manufacturing standards.